Follow Us @
EMA Europa to start review on SINOVAC Vaccine
The European vaccine regulator, the EMA, will watch the trials of the corona vaccine of the Chinese state-owned company Sinovac. This so-called rolling review is the last step before the developers request approval and Europe decides whether the vaccine can be marketed.
EMA has decided to initiate this process based on preliminary investigation results. “These suggest that the vaccine will trigger the production of antibodies targeting the coronavirus and may help protect against the disease.”
The regulator will review the data that is already available about the vaccine, while it is still in the final research phase. When enough data is available to complete the research phase, the rolling review will stop .
By looking at the data during the ongoing research, the vaccine may be assessed more quickly. If the EMA then considers the vaccine to be effective and safe, it could possibly be used in Europe.
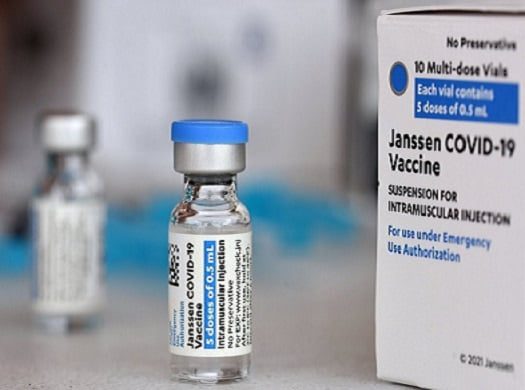
Vaccine appears to be less effective
Figures on the effectiveness of the Chinese vaccine vary depending on where the research was conducted. Immunologist Marjolein van Egmond (Amsterdam UMC) told NU.nl on Monday that this starts at 50 percent, but sometimes turns out to be much higher.
“Overall it appears to be less effective than RNA vaccines, such as Pfizer and Moderna, but it is still effective in preventing people from becoming seriously ill and ending up in hospital. Overall, it is still a very useful vaccine” , the immunologist concluded.
So far, there have been no contract negotiations from the Netherlands regarding the purchase of the Chinese vaccine.
MORE@AFRILATEST
JOIN US ON FACEBOOK EMA Europa
-

 Fashion3 months ago
Fashion3 months agoVogue Arabia cover welcomes Salma Hayek in an interview with Penélope Cruz
-

 Football3 months ago
Football3 months agoVAR points out Diego Costa's offense against the fourth referee
-

 USA today entertainment3 months ago
USA today entertainment3 months agoBeyonce with the single “Break My Soul” leads on Spotify Brazil
-

 Health and Fitness3 months ago
Health and Fitness3 months agoVaccine against the reappearance of skin cancer enters final testing phase
-

 USA today entertainment3 months ago
USA today entertainment3 months agoSZA, Future and DJ Khaled come together in collaboration
-

 News3 months ago
News3 months agoParents of former player Waleswska are pressured by widower to pay rent for the house where they live
-

 USA today entertainment3 months ago
USA today entertainment3 months agoLarissa Luz and Linn da Quebrada enchant at the Multishow Awards with a tribute to Elza Soares.
-

 Good News TV series3 months ago
Good News TV series3 months agoThe shocking reason behind the decision not to show dead characters in The Last Of Us episode revealed