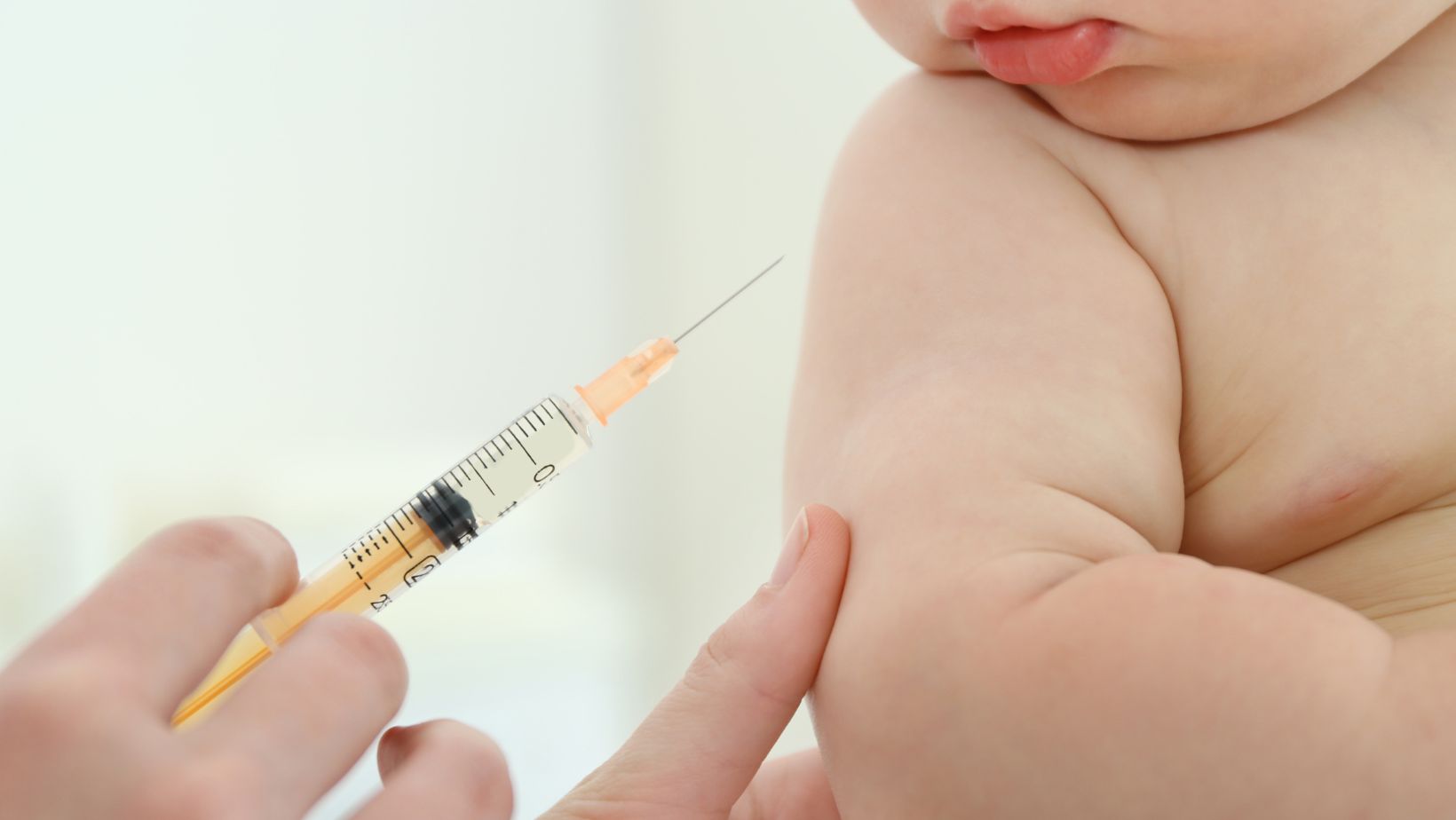
Modern vaccine now available for 6-month-old babies in Brazil
Follow Us @
Modern vaccine now available for 6-month-old babies in Brazil
This last Monday (26), Anvisa (National Health Surveillance Agency)approved the first registration of the vaccine bivalent spikevax of Moderna against Covid-19 in Brazil. It protects against both the original strain and the omicron variant. will be distributed by Adium Group.
The drugmaker plans to extend the immunization authorization for children from 6 months. Until now, vaccination was approved only for adults and children up to six years old. This expansion in authorization is intended to provide an additional layer of protection against the coronavirus and its variants, especially among the most vulnerable groups. However, it is important to emphasize that immunization in infants aged 6 months will be administered in an initial two-dose schedule, ensuring an adequate immune response.
The Adium Group, responsible for operating the vaccine in Brazil, reported that it intends to bring it to the public and private network. “There is still a lack of regulatory rituals, about the price of the vaccine, for example. Brazil is a priority for modern declared Adium’s regional medical director for vaccines in Latin America, Glaucia Vespa, together with Moderna.
According to Thiago Barbosa, director of Adium’s vaccine unit in Brazil, the pharmaceutical group has revealed its interest in entering the Brazilian market since January last year, however, the delay is related to global regulatory changes. “We thought it best to position the vaccine associated with the omicron variant. So we wait to offer an adapted vaccine. We will have the vaccine with strains BA.4 and BA.5.”,informs the director. There is still no set deadline for the arrival of doses in Brazil, nor established price. It is already administered in 38 countries and has also been approved by the European Medicines Agency. (EMA) and the Food and Drug Administration (FDA)regulatory agency of the United States.
Featured photo: Anvisa authorizes Moderna’s bivalent Spikevax vaccine in Brazil/Mike Segar/Reuters/Reproduction
Modern vaccine now available for 6-month-old babies in Brazil
Follow AFRILATEST on Google News and receive alerts for the main news about celebrities, soap operas, series, entertainment and more!
SHARE POST AND EARN REWARDS:
Join our Audience reward campaign and make money reading articles, shares, likes and comment >> Join reward Program
FIRST TIME REACTIONS:
Be the first to leave us a comment, down the comment section. click allow to follow this topic and get firsthand daily updates.
JOIN US ON OUR SOCIAL MEDIA: << FACEBOOK >> | << WHATSAPP >> | << TELEGRAM >> | << TWITTER >
#Modern #vaccine #6monthold #babies #Brazil
-
News2 months ago
Harry decides to appeal after loss of police protection in the UK
-
Good News TV series2 months ago
Preta Gil is the new presenter of TVZ on Multishow
-
Good News TV series2 months ago
Justin Bieber and Hailey go to church using a powerful car
-
Good News TV series2 months ago
Fuzuê: Pascoal is sentenced to more than 70 years in prison
-
Health and Fitness2 months ago
Hyperbaric Oxygenation accelerates recovery from knee injuries, according to USP study
-
Culture3 months ago
the Sub4 Turismo package that we recommend and we go (on the 42 km)
-
News3 months ago
Viradouro wins the Rio de Janeiro Carnival title in 2024 now news
-
Health and Fitness3 months ago
See tips for getting pregnant after age 40